Understanding challenges of malaria vaccination, as elimination becomes achievable
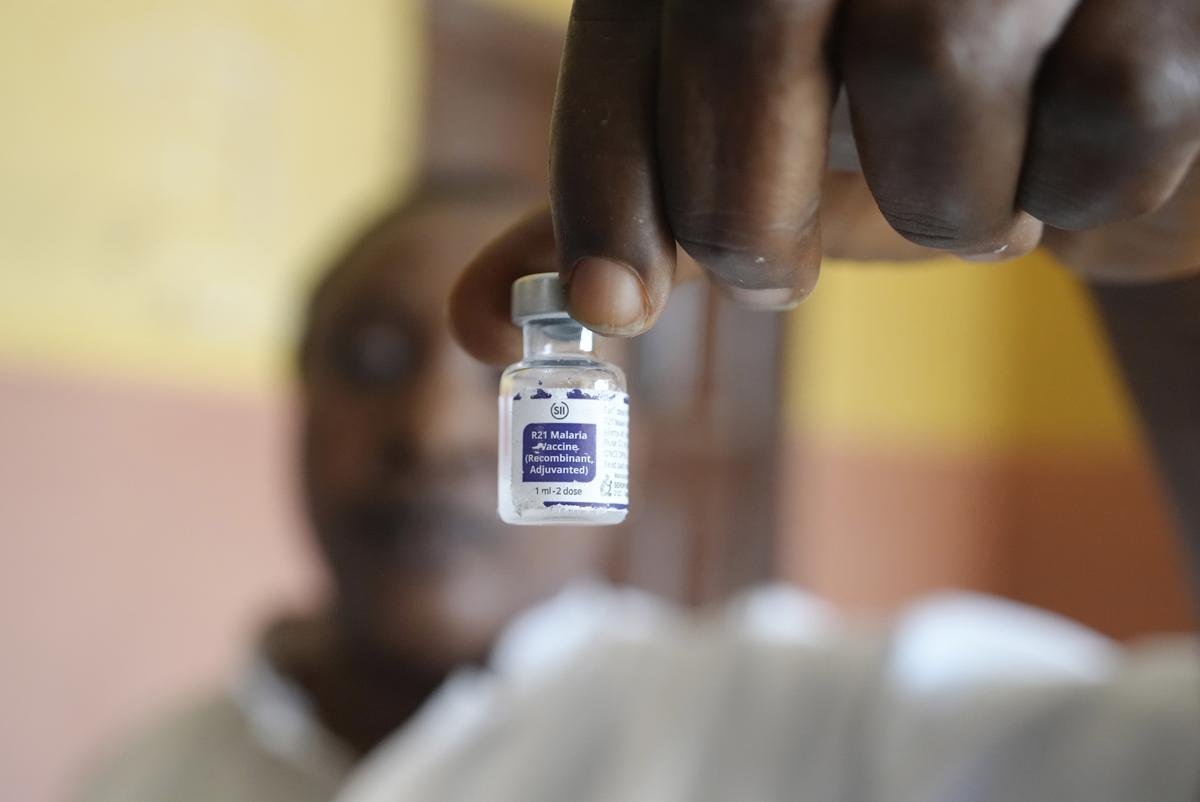
The R21/Matrix-M vaccine enhances the immune response with a stronger adjuvant and has shown 77% efficacy over 12 months.
The WHO’s recent declaration of Georgia as the 45th country to eliminate malaria is a significant milestone. Yet, even as we celebrate this achievement, a lingering question remains: if smallpox has been eradicated, why does malaria persist, and why is its vaccine far less effective than those for viral diseases? Despite decades of global efforts, malaria still causes over 240 million cases and more than 600,000 deaths annually.
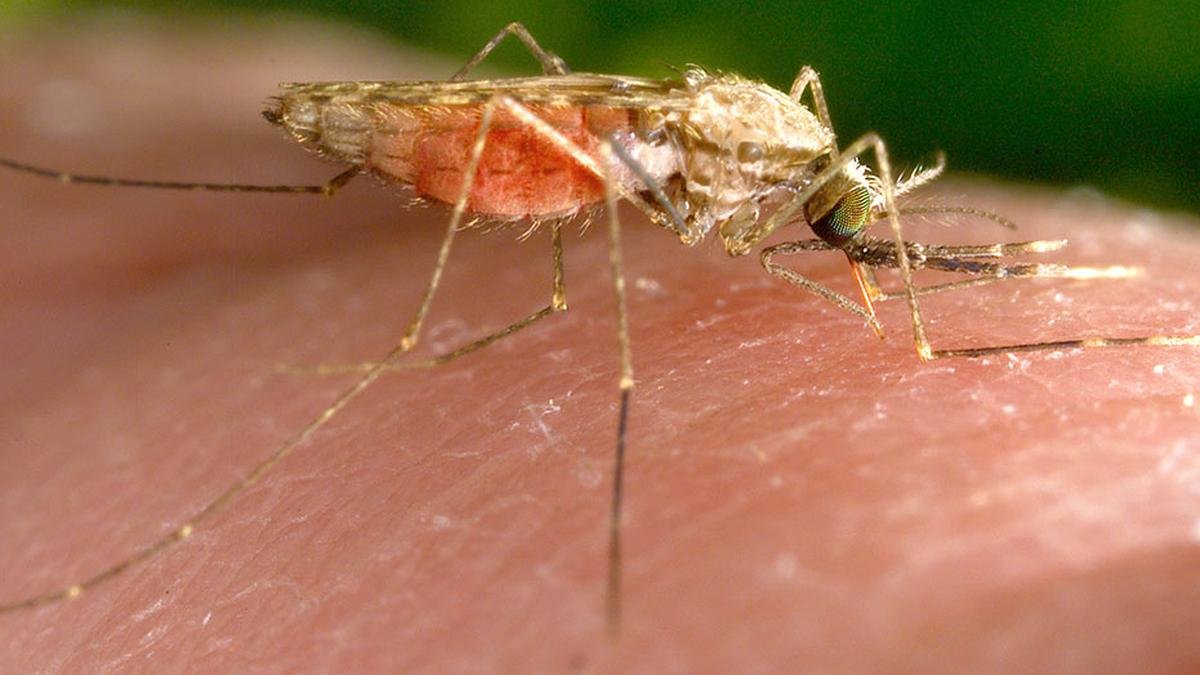
The discovery of malaria’s transmission was a fragmented journey, like a non-linear movie where different revelations come together to complete the puzzle at the end. Malaria was believed to be caused by miasma or foul air from swamps. This misconception persisted until Alphonse Laveran, a military doctor, identified the Plasmodium parasite in 1880, proving malaria was caused by a living organism. However, the question of how the parasite entered humans remained unanswered. In 1891, Patrick Manson hypothesised that mosquitoes played a role in transmission, though he lacked experimental proof.
Giovanni Grassi later confirmed that only female Anopheles mosquitoes carried the parasite, but the full cycle was still unclear. The final breakthrough came in 1897 when Ronald Ross in India demonstrated that Plasmodium completed its life cycle in mosquitoes, proving they were malaria’s vectors. This scientific triumph had far-reaching consequences, allowing European colonial powers to survive in tropical Africa, where malaria had previously limited their expansion. In 1870, only 10% of Africa was under European control, but by 1914, nearly 90% of the continent had been colonised. Ironically, instead of liberating the populations most affected by malaria, the discovery of its transmission pathway paved the way for their subjugation, reinforcing colonial rule rather than dismantling it. Progress in science does not always translate to moral or equitable outcomes.
Understanding parasites
Knowing the parasite’s life cycle is essential to understand the difficulty in developing vaccines. The cycle begins when an infected Anopheles mosquito bites a human, injecting Plasmodium sporozoites that are highly infective, into the bloodstream. These parasites first travel to the liver, invading liver cells and multiplying undetected by the immune system. After this phase, they re-enter the bloodstream, infecting red blood cells and causing malaria’s characteristic fever and chills. As the parasite multiplies asexually within RBCs, some develop into sexual forms known as gametocytes, which are then taken up by another mosquito when it bites an infected individual. Inside the mosquito, these gametocytes undergo sexual reproduction, maturing into sporozoites that migrate to the mosquito’s salivary glands, making the mosquito infectious to new human hosts.
Viruses are simpler in comparison, consisting of only genetic material (DNA or RNA) encased in a protein shell, and are relatively straightforward compared to parasites. Plasmodium is a protozoan parasite and eukaryotic organism with multiple stages of development, each featuring different surface antigens. Plasmodium species that infect humans — P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi have unique characteristics, adding to the challenge of vaccine development.
The malaria parasite masters deception, outmanoeuvring the human immune system. Its greatest strength is antigenic variation, where it frequently changes its surface proteins, making it difficult for immune cells to recognise it and respond. Plasmodium follows an intracellular lifestyle, hiding within the liver and RBCs, shielding itself from immune surveillance. This ability to evade detection weakens the body’s ability to develop long-lasting immunity, making reinfection common. Adding to the challenge, malaria’s life cycle spans multiple stages across two hosts—humans and mosquitoes—requiring any potential vaccine to target several phases simultaneously. So when a vaccine is developed, the parasite’s genetic adaptability enables it to evolve resistance.
Fighting malaria is like trying to corner a cunning politician who changes allegiance and ideology, senses the public sentiment, and always stays ahead of the game to remain in power. Plasmodium has been perfecting this trick for 30 million years, adapting and evolving to ensure its survival. When scientists find a way to combat one strain, the parasite shifts tactics, altering its proteins and genetic structure to bypass immunity. The adaptability makes it difficult to develop a universal and long-lasting vaccine.
Malaria vaccines
The RTS, S malaria vaccine became the first to receive WHO approval for large-scale rollout in endemic regions after 60 years of research. It targets the liver stage of the parasite’s life cycle by inducing an immune response against the circum-sporozoite protein (CSP) found on Plasmodium sporozoites. This stage infects the liver after a mosquito bite. However, its efficacy is limited, reducing malaria cases by approximately 36% after four doses over four years in children. It is significantly lower than the 90-95% efficacy in vaccines for diseases like measles. Protection is also inconsistent, varying across age groups and transmission settings, and its effectiveness declines over time. RTS, S requires multiple doses, posing logistical challenges ensuring widespread distribution and coverage in resource-limited regions.
Researchers are now developing second-generation malaria vaccines. One promising candidate is the R21/Matrix-M vaccine, which enhances the immune response with a stronger adjuvant and has shown 77% efficacy over 12 months. The PfSPZ vaccine, a whole-parasite vaccine, attenuates sporozoites to stimulate immunity against the liver stage of infection. RH5-based vaccines prevent Plasmodium from invading red blood cells and target the blood stage after symptoms. Meanwhile, transmission-blocking vaccines aim to halt the spread of malaria by preventing mosquitoes from becoming carriers, targeting proteins like Pfs25 and Pfs230, essential for parasite development within the mosquito.
Research underfunded
Malaria vaccine research has long suffered from underfunding due to geopolitical and economic factors. Since malaria primarily affects low-income countries in Africa and parts of South Asia, funding for research and healthcare infrastructure has remained limited. The availability of treatments for malaria has reduced the immediate urgency for vaccine development. Compounding this, the complexity of the parasite itself has discouraged pharmaceutical companies from investing in malaria vaccines, as the cost of research is high, and potential returns on investment remain uncertain. Countries are now facing a resurgence of the disease due to shifting mosquito habitats. An effective malaria control strategy will require a combination of more efficient vaccines, mosquito control strategies & improved treatment options. The road to a malaria-free world is long, but it is an achievable goal.
Published – February 13, 2025 11:04 pm IST


Post Comment